Why can graphite be made into conductive materials?
xinstNov 19, 2020
Everyone should know about the conductivity of graphite anodes, but why does graphite conduct electricity? How can it be made into conductive materials?
Because graphite contains freely moving charges, the charges move freely after being energized to form a current, so it can conduct electricity. The real reason why graphite conducts electricity is that 6 carbon atoms share 6 electrons to form a large ∏66 bond with 6 electrons and 6 centers. In the same carbon ring of graphite, all 6-membered rings form a ∏-∏ conjugate system. In other words, in the same carbon ring of graphite, all carbon atoms form a huge big ∏ bond, and all electrons in this big ∏ bond can flow freely in the layer. This is the reason why graphite paper can conduct electricity.
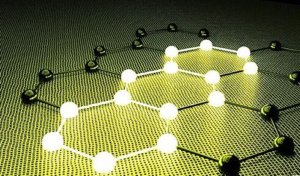
Graphite has a lamellar structure with unbonded free electrons between the layers, which can move directionally after being energized. In fact, all materials conduct electricity, it is only a matter of resistivity. The structure of graphite determines that it has the smallest resistivity among carbon elements.
Graphite anodes are conductive, and graphite particles made of graphite are of course conductive, but they will have different effects if they are made differently. What should I say, because there are two kinds of graphite particles, one is like a new Small graphite particles like collagen, with a particle size greater than 0.5 mm, which is almost the coarsest powder crushed by a pulverizer. The other one is made of high-purity graphite. Any specification can be made. High purity Graphite is characterized by high purity of graphite, which restores the original physical properties of graphite. At the same time, graphite products of different shapes can be produced, such as graphite electrode rods that require conductivity, graphite lubricating rods, graphite sheets for heat dissipation, and various small-size graphite particles. These have the unique characteristics of graphite such as conductivity, lubrication, and high temperature resistance. High-purity graphite is impregnated after roasting. The immersion at 1800 degrees has completely volatilized the binder used in the manufacture of high-purity graphite materials, which highlights the conductivity of graphite, because the higher the purity of graphite, the better the conductivity. .

